February.17.2025

NEW Training Session at EPISKIN Facilities
On March, 10 & 11, 2025 (full)
On June, 16 & 17, 2025.
Limited numbers of trainees, if interested please suscribe here: Training Session Form
January.10.2022
 Adebiotech General Assembly
Adebiotech General Assembly
Dr Christian Pellevoisin, Scientific Director of the EpiSkin Academy will do a short presentation during the Adbiotech General Assembly on January 17th.
Complete program:
10h00 –Welcome
Manuel GEA, Président Adebiotech
10h10 – General conference
Dominique HELAINE, Suez
Carbon issues for biotech companies linked to the climate challenge
10h30 – Adebiotech members' presentations
Rozenn RAVALLEC, Institut Charles Viollette
10h40 – Christian PELLEVOISIN, EPISKIN
EPISKIN, leader in skin engineering for animal-free evaluation
10h50 – Maxence DESJONQUÈRES, BIOMILLENIA
11h00 – Pascale BESSE HOGGAN, Université Clermont Auvergne
11h10 – Francis FINOT, GenEvolutioN
11h20 – Robin DUMAS, Vibiosphen (au nom de Maxime FONTANIÉ, Vibiosphen)
11h30 – Jérôme CAUSSIN, Novozymes
11h40 – Review and projects Adebiotech
Danielle LANDO et Sylvio BENGIO, Adebiotech
12h10 – General discussion of projects
12h20 – General Assembly
- Moral report
- Balance sheet
- Election of the new Board of Directors
To attend the event please follow this link: https://asso.adebiotech.org/ag-2021/
December 16.2021
 ADEBIOTECH Think Tank
ADEBIOTECH Think Tank
After the success of the ADEBIOTECH conference on December 9th, EPISKIN decided to join ADEBIOTECH to support this Think Tank which allows to cross the expertise from several industrial fields, pharmaceutical, cosmetic, food, agrochemical...
To learn more about ADEBIOTECH Think Tank: https://asso.adebiotech.org/

November.10.2021
 EPISKIN sponsors the 4th Annual Conference of SAAE-India (SAAE-I AC-4): Latest Trends in ‘Organ culture & Organs on Chip’ Technologies, 11Tth & 12th December 2021.
EPISKIN sponsors the 4th Annual Conference of SAAE-India (SAAE-I AC-4): Latest Trends in ‘Organ culture & Organs on Chip’ Technologies, 11Tth & 12th December 2021.
The 4th Annual Conference of the SAAE-I is being organized with an aim to bring together stakeholders of 3’Rs - academia, scientific community, industry, government and animal behaviour / welfare personnel from national /international levels to raise awareness / facilitate exchange of information / ideas on alternatives.
This conference is being organized in an online mode under the aegis of Delhi Pharmaceutical Sciences and Research University (DPSRU), established by the Act7 of 2008 of State Legislature of Delhi as a State University, the first Pharmacy University of the Indian subcontinent. DPSRU is rapidly emerging as an ultimate destination for education, training and research in pharmaceutical and allied health sciences. The DPSRU has around 1300 students and around 150 staff members. It is equipped with the State-of-the-art equipped Centre of Excellence and is producing top class pharmaceutical scientists, pharmacists, and health professionals thereby significantly contributing to the development of Pharmaceutical Sciences across the globe in general, and India in particular.
Scientific program:
- Plenary expert talks
- E-posters and oral presentations by young scientists & scholars
- Panel discussions and networking opportunities
- Regulatory trends based on the 3R’s
- “Loreal – Episkin” Awards for best oral and poster presentations to early carrier researchers/scientists
- Post-conference workshops on:
- Reconstructed human tissues (Dr. Christian Pellevoisin, Episkin, France),
- Integrated discrete Multiple Organ Co-culture (IdMOC) (Dr. Albert P. Li, AP Sciences Inc, Columbia, MD, USA),
- Drosophila culture and analysis (Dr. Suhel Parvez, Jamia Hamdard, New Delhi)
- C. elegans culture and analysis (Dr. Aamir Nazir, CDRI, Lucknow)
Speakers:
- Dr. Thomas Hartung - CAAT, Johns Hopkins, USA
- Dr. Lena Smirnova - CAAT, Johns Hopkins, USA
- Dr. Adrian Smith - NORCOPA, Norway
- Dr. Francois Busquet - AlterTox, Belgium
- Dr. Hajime Kojima - NIHS, Japan
- Dr. Christian Pellevoisin - Episkin, France
- Dr. P.V. Mohanan - SCIMST Trivandrum
- Dr. A.B. Pant - CSIR-IITR, Lucknow
- Dr. Jaya sujatha - Unilever, UK
- Dr. Albert P. Li - AP Sciences Inc, USA
- Dr. Suhel Parvez - Jamia Hamdard, New Delhi
- Dr. Aamir Nazir - CDRI, Lucknow


March.30.2021
 Ingéniérie tissulaire, modèles de tissus en 3D pour l’industrie
Ingéniérie tissulaire, modèles de tissus en 3D pour l’industrie
EPISKIN supports ADEBIOTECH's Webinar on March 30th 2021 on Tissue Engineering for the Industry. Their application in Cosmetics, Pharmacology, Chemistry, Nutrition.
Please register to the webinar following this link: https://asso.adebiotech.org/webinaires/ingenierie-tissulaire-modeles-3d-pour-lindustrie-cosmetique/
February.2.2021
 FIRST EPISKIN ACADEMY WEBINAR, TUESDAY, FEBRUARY 02, 2021 02:00 PM CET
FIRST EPISKIN ACADEMY WEBINAR, TUESDAY, FEBRUARY 02, 2021 02:00 PM CET
ISO 10993-23 FOR BIOCOMPATIBILITY OF MEDICAL DEVICES
The new ISO 10993-23 for bio-compatibility of medical devices introduces the shift from in vivo to in vitro for the assessment of irritation potential of medical devices.
This is a change in mindset for which industry needs to prepare. The EPISKIN Academy webinar will cover irritation of medical devices, the scientific reasoning, the added value and the current limits of this new strategy. The training part of the Webinar – live broadcasting of the hands-on part of the assay - is a unique opportunity to discover all the tips and tricks of this protocol through an in laboratory session covering all the steps of the protocol.
Webinar’s agenda
Introduction (5 minutes)
Alain Alonso – Sales and Marketing Director &
Christian Pellevoisin - Scientific Director of EPISKIN Academy
EPISKIN – Presentation of the Company (5 minutes)
Alain Alonso – Sales and Marketing Director
Production and Quality Controls (5 minutes)
Anne-Sophie RIGAUDEAU – Production Director
ISO 10993-23, a shift from in vivo to in vitro assays for irritation of medical devices (25 minutes)
Christian Pellevoisin – Scientific Director of EPISKIN Academy
Live Demonstration of the in vitro assay for medical devices with 3D RECONSTRUCTED MODELS (10 minutes)
Christelle Videau - Scientific Customer Experience Manager
Q&A (30 minutes)
03:30 pm: End of the 1st EPISKIN Academy Digital Event
Hope to welcome you soon on our 1st websession online!
July.15.2020

Oral communication “Status update of new requirements ISO 10993 23” at MedTech Summit
The 2020 edition of the MedTech Summit will be on “EU MDR & IVDR: 2 YEARS TO GO. 2 REGULATIONS TO MEET. ARE YOU READY?”
Due to Covid-19 crisis this edition goes virtual. It will be held from October 12th to 16th, 2020.
Friday, 16 October 2020 15:00 - 15:25: Dr Christian Pellevoisin, Scientific Director of EPISKIN Academy, will present during the Biocompatibility for Medical Devices session:
Status update of new requirements ISO 10993- 23 ISO 10993-23 is a new standard expected this fall that will introduce the shift from in vivo to in vitro for the assessment of irritation potential of medical devices.
This is a change in mindset for which industry needs to prepare. The convenor of ISO/TC 194/WG 8 and chairman of AFNOR S92/J for the biocompatibility of medical devices will present the scientific reasoning, the added value and the current limits of this new strategy.
To discover how to subscribe to this event and full programme, please follow this link: MedTech Summit
April.30.2020
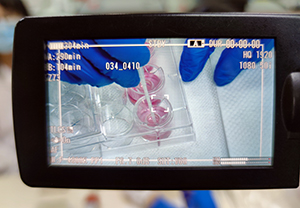
First live-broadcasting training event by Shanghai EPISKIN
Despite the impact from COVID-19 outbreak and restrictions on travel and conference gathering, the 9th EPISKIN Academy Workshop has just been successfully carried out through the live-broadcasting platform on Apr. 17th at Shanghai EPISKIN’s head office in Zhangjiang Hi-Tech park. This is also our FIRST online live-streaming show of technical training and product support to meet in vitro alternative testing related needs.
In our initiative to quickly adapt to the current circumstance with the agility to timely respond to our clients’ requests on technical training and support, given the fact that working activities cross sectors are recovering in China but regular on-site training still cannot be organized for the moment, this online workshop highly demonstrated the efficiency and effectiveness of such a new format for technical training and support.
Content of this 9th EPISKIN Academy Online Workshop:
1) Opening and Introduction
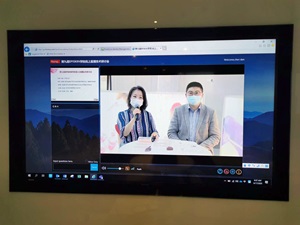
2) Review of in vitro Skin Irritation EpiSkin testing method (OECE TG439)
3) Demonstration of key technical steps of Skin Irritation Test using EpiSkin model and data analysis

4) Introduction of 3D skin models commercialized in China
5) Discussion Q&A

During the very special period we are all going through around the world, EPISKIN and the EPISKIN Academy are experimenting new way of communication using more and more digital technologies. This totally new way of sharing skin irritation protocols by live-broadcasting is a great success and we will soon provide other live sessions all over the world.
March.03.2020
 EPISKIN Academy at Nelson Labs seminar
EPISKIN Academy at Nelson Labs seminar
Dr. Christian Pellevoisin, Scientific Director at Episkin Academy, will present 'Assessing Alternatives to In-Vivo testing: Changing the Paradigm from In-Vivo to In-Vitro Toxicology' during the Nelson Labs seminar in Brabanthal, Leuven, Belgium, March 4 & 5, 2020.
Scientific program:
Leuven Open House 2020: The Year Of Change for the Medical Device Industry
Wednesday, March 04, 2020
12:00 Registration & welcome
13:10 Introduction – Eric Meyers (Senior VP EMEAA Nelson Labs) & Jos Bollen (Managing Director Nelson Labs Europe)
13:30 The EU Medical Device Regulation (MDR): 2020: The Year of Change – Henry Sibun (Henry Sibun Associates Ltd)
14:00 The new ISO 10993-18 Standard and its Impact on Chemical Characterization of Medical Devices – Dr Ted Heise (Vice President Regulatory and Clinical Services at MED Institute)
14:30 Linking ISO 10993-18 to ISO 10993-17 – Dr. Carsten Baun Senholt (ERT Chief Technical Officer at Saxocon)
15:00 Coffee break
15:15 MDR – View of a Raw Materials Supplier - Steve Duckworth (Global Head Marketing and Business Development at Clariant)
15:45 ISO 10993-18 in the MDR: understanding the restrictions and risk assessmentfor compounds which are carcinogenic, mutagenic, toxic to reproduction (CMR) or have endocrine-disrupting (ED) properties (section 10.4) – Dr. Annelies Vertommen (Senior E&L Expert Nelson Labs Europe)
16:15 Tour of the Laboratory Facility
17:00 Networking event
19:00 End of Seminar Day 1
Thursday, March 05, 2020
08:30 Registration
09:00 Introduction (Nelson Labs Europe)
09:05 MDR – Biocompatibility & reprocessing - Dr Gert Bos (Executive Director & Partner at Qserve)
09:45 The Biological Evaluation Plan (BEP): A crucial first step in the Biocompatibility evaluation of a Medical Device – Dr Sophie Michel (Associate Biocompatibility Expert)
10:15 Competent Authority perspectives on biocompatibility – Dr Pieter Van De Vijver (Non-Clinical Assessor, Medical Devices at FAMHPS)
10:45 Coffee Break
11:15 Assessing Alternatives to In-Vivo testing: Changing the Paradigm from In-Vivo to In-Vitro Toxicology – Dr. Christian Pellevoisin (Scientific Director at Episkin Academy, L’Oréal)
11:45 The need to Identify “Unknowns” from a Risk Management Perspective – Ron Brown (ex-FDA; now Director at Risk Science Consortium LLC)
12:15 Lunch
13:30 How to select a CRO for Chemical Characterization Testing – Dr Dennis Jenke (Principal Consultant Nelson Labs Europe)
14:00 Most Common Types of Observations in Regulatory Submissions of Chemical Characterization Results and how to address them – Dr. Piet Christiaens (Scientific Director at Nelson Labs Europe)
14:30 Break
15:00 Intro The Processing Cycle – Henry Sibun (Henry Sibun Associates Ltd)
15:15 Sterilization of your Medical Device – Annick Gillet (Technical Director EO Pharma – Sterigenics)
15:45 Reprocessing Validations of reusable Medical Devices (part 1) – Dr Lise Vanderkelen (Department Head Micro & Pharma Services Nelson Labs Europe)
16:15 Reprocessing Validations of reusable Medical Devices (Part 2) – Alpa Patel, Principal Scientist (Nelson Laboratories LLC)
16:45 Q&A (panel)
17:00 End of Seminar Day 2 – tour of the laboratory facility on-demand
*Times and topics subject to change
Direct link to Nelson Labs Seminar page:
www.nelsonlabs.com/
February.03.2020

Workshop on ocular irritation in Brazil
This Workshop will be the first complete training session on ocular irritation on SkinEthic HCE model for OECD TG492 and OECD TG437 in Brazil.
This program in partnership with InMetro follows the first training session in 2017 on skin irritation: https://www.youtube.com/watch?v=_daWZdYxh6Q
The workshop will be held in Inmetro, Campus de Metrologia e Inovação from February 03 to 07, 2020.
November.07.2019

ISO 10993: Are you biocompatible?
Tackle Testing Methods, Evaluations, Risk and Regulations With Competent Authority, Notified Body and Industry Guidance.
Dr Christian Pellevoisin from EPISKIN Academy will do a presentation on November 21st, CCIB, in Barcelona, Spain.
Assessing alternatives to animal testing: Changing the paradigm from in vivo to in vitro toxicology:
- Examining alternative available methods to predict biocompatibility
- Strategies for demonstrating the value of in vitro methods to predict biocompatibility
- Validation of skin irritation on 3D models to replace animal testing
- Latest developments in skin sensitization and perspectives for biomedical devices
- Assessing the need for positive materials with good human data to validate existing assays in vitro
Presentation will be held from 09:10 to 09:45.
If you want to learn more about presentation and book this session you can check website:
https://lifesciences.knect365.com/biocompatibility/speakers/christian-pellevoisin
October.16.2019
 Conference on Biocompatibility for Medical Devices US
Conference on Biocompatibility for Medical Devices US
The EPISKIN Academy, represented by Dr Christian Pellevoisin, will do a presentation during Biocompatibility for Medical Devices US.
October 24, 2019 in JW Marriott Chicago, Chicago, IL, USA.
Examining the latest developments in skin sensitisation and irritation testing:
- Validation of skin irritation on 3D models to replace animal testing
- Assessing how far away the industry is from having positive materials with good human data
- Assessing the need for good human data to validate existing assays in vitro
- Defining the right strategy for sensitisation and irritation testing
- Materials are on the market with no complaints/PMS requirements
Thursday, 24 October 2019 3:15pm - 3:50pm
To read more about the Congress: https://lifesciences.knect365.com
September.17.2019
 First International Forum on Quality Evaluation of Medical Devices and Biomaterials
First International Forum on Quality Evaluation of Medical Devices and Biomaterials
The Shandong Medical Device Product Quality Inspection Center held a lecture on the quality and safety evaluation of medical devices and biomaterials and the first international forum on quality evaluation of medical devices and biomaterials to further promote the improvement of the quality evaluation of biomaterials and medical devices, and promote the industry.
The first International Forum on Medical Device and Biomaterial Quality and Safety Evaluation jointly sponsored by Shandong Materials Biology Evaluation Branch and China Biomaterials Society will be held in Jinan, Shandong Province in September 2019.
This forum will bring together experts and scholars from different domestic and international review and approval, inspection and testing, research institutions and industrial enterprises to evaluate the quality of medical devices, focusing on the chemical characterization of medical devices and biological materials, biological evaluation and quality around medical devices.
Safety evaluation related to pre-clinical large animal experiments, integrating the three major cities of production, learning, research, the application of the latest hot topics, research progress and technical difficulties, such as academic reports, experience exchange and technology display.
In order to promote the development of medical device biomaterials quality and safety in the new situation, the new power of the committee will gather in Jinan to participate in the academic event!
September 23-25, 2019: Jinan Yinhuada Hotel ( No.1, Lvxi Caidang, Longxi Road, South Lixia District, E1, 0,531,058,999).
Download Chinese Meeting announcement here.
November.14.2018
EPISKIN Academy at the National Conference on Alternatives to Animal Experiments
EPISKIN Academy is invited to give a talk and a pre-conference training to the National Conference on Alternatives to Animal Experiments (NCAAE-2018) that will be held December 1st 2018 in New Delhi (India).
The Conference will be held at Jamia Hamdard, New Delhi, a Deemed to be University accredited in category ‘A’ by the National Assessment and Accreditation Council of India.
This Conference aims to discuss the perspectives on the current state of practices and existing strategies to promote the development of non-animal technologies in India (http://www.jamiahamdard.ac.in/NCAAE2018/NCAAE.htm)
On November 26th, Jamia Hamdard in collaboration with EPISKIN Academy under the aegis of Indian Society for Alternatives to Animal, organize a Hands-on Workshop to train 20 young scientists on Alternatives and in vitro skin Irritation according to OECD TG-439.
(http://www.jamiahamdard.ac.in/NCAAE2018/Hands-on%20workshop%20on%20reconstructed%20human%20epidermis_OECD%20TG%20439_Jamia%20Hamdard.pdf)
October.25.2018

EPISKIN at the Eurofins Human Safety Seminar
Come and discover the new EPISKIN Academy presentation during the ESTIV congress:
Thursday, ocotber 18th
09.18 (OP 32):
Reconstructed human epithelia for in vitro biocompatibility of medical devices
Pellevoisin Christian (Lyon/FR)
To discover full programme please visit the ESTIV website.
May.23.2018.

|
The EPISKIN Academy at the IID 2018
|
During last IID (International Investigative Dermatology) congress which was held from May 16th to 18th, in Rosen Shingle Creek Resort, Orlando, Florida, the EPISKIN Academy presented two posters about the EPISKIN models:
01156 T-SKIN, a new industrial reconstructed human skin model for dermatological and cosmetics research and development
C. Pellevoisin, D. Lelièvre, M. Bataillon, N. Boyera, A. Rigaudeau, J. Ovigne, I. Besné, N. Seyler
Journal of Investigative Dermatology, Vol. 138, Issue 5, S196
Published in issue: May 2018
https://doi.org/10.1016/j.jid.2018.03.1170
01085 Cellular mechanistic investigation on antigen delivery by Viaskin® patch for epicutaneous immunotherapy with reconstructed human epidermis including Langerhans cells (SkinEthicTM RHE-LC)
V. Dioszeghy, C. Pellevoisin, M. Ligouis, F. Sahuc, V. Dhelft, L. Mondoulet
Journal of Investigative Dermatology, Vol. 138, Issue 5, S184
Published in issue: May 2018
https://doi.org/10.1016/j.jid.2018.03.1098
March.29.2018.
 |
EPISKIN ACADEMY poster reward at the 57th SOT
|
L'Oreal China and Shanghai EPISKIN Biotechnology Ltd. announced the creation of the EPISKIN Academy in China.
Through training and technical collaborations, the Academy will work with Chinese scientists, key opinion leaders, students and future stakeholders in terms of scientific and regulatory issues for alternatives to animal testing in field of safety, toxicology and efficacy as well as in other relevant areas.
To further promote the reconstructed skin models through marketing, L'Oréal China and EPISKIN SA created Shanghai EPISKIN Biotechnology Ltd. in 2014 to produce reconstructed skin models according to high quality standards in the industry. The models will be used primarily for research on skin tissue engineering and testing. This time the creation of the EPISKIN Academy will certainly play an important role in promoting in vitro skin models in the industry.
"In China, the Academy will work closely with various parties to promote the reconstructed skin models and alternative methods in China, and will strive to become a key scientific resource to further stimulate innovation in the Chinese cosmetics industry" said Dr. Christian Pellevoisin, scientific Director of the EPISKIN Academy.
Dr Christian Pellevoisin, Scientific Director of the Episkin Academy, has been invited by the Organizing Committee of CIFARP to be a speaker at 10th Congress of Pharmaceutical Sciences that will be held in Ribeirão Preto – São Paulo -Brazil, from September 06-09, 2015.
This year the theme is “Translating science into health pipeline” :
September 8th - 10:30-12:30 - S9.2: Episkin and the application of reconstructed human 3D models in toxicology and pharmacology.
CIFARP 2015 will be a unique opportunity to discuss the latest trends and scientific breakthroughs in the pharmaceutical sciences field. About 1400 national and international participants are expected, including researchers, professionals, as well as postgraduate and undergraduate students.
For more information consult the website of the congress: http://www.cifarp.com.br/scientific-program.php